FDA Action on CBD Remains TBD As Producers Bide Time

By William Sumner, Hemp Content Manager, New Frontier Data
The CBD industry is struggling: Amid disruption by the COVID-19 outbreak and supply-chain shortages, the industry also finds itself mired in regulatory uncertainty due to a ponderous attitude by the U.S. Food and Drug Administration (FDA) in regulating CBD. At the heart of the issue for the FDA is whether CBD should be allowed as a dietary supplement or limited as a pharmaceutical ingredient.
The FDA approval process for pharmaceutical drugs is administratively arduous, taking multiple clinical trials, millions of dollars invested, and several years to complete. Even at the end of its long process, there is no guarantee that the FDA will grant approval. Currently, there is only one CBD-based pharmaceutical drug on the market, Epidiolex, an anti-epilepsy medicine manufactured and patented by GW Pharmaceuticals.
Under the Dietary Supplement Health and Education Act of 1994 (DSHEA), dietary supplements are defined as a products intended to supplement the diet and containing one or more dietary ingredients for use by humans to supplement their diets.
Like the European Union (EU)’s novel food classification, any dietary supplement marketed in the U.S. prior to 1994 does not require FDA approval. Those substances not grandfathered in, like CBD, must provide reasonable evidence or expectations that the product is safe to use prior to gaining market approval.
As outlined by DSHEA, companies selling or marketing new dietary supplements must notify the FDA at least 75 days prior to bringing the product to market. Though some regulatory approval is required, that is still a significantly shorter timeline than the pharmaceutical approval process.
For its part, the FDA finds itself between a rock and a hard place. On the one hand, there is already massive consumer interest in CBD. New Frontier Data estimates that approximately 73 million Americans will purchase CBD by the end of 2020.
On the other hand, the FDA is concerned about the safety of CBD. In an update to consumers, the FDA outlined many of its concerns regarding CBD, including how the substance interacts with other drugs, its effect on liver function, and the long-term effects of continued use.
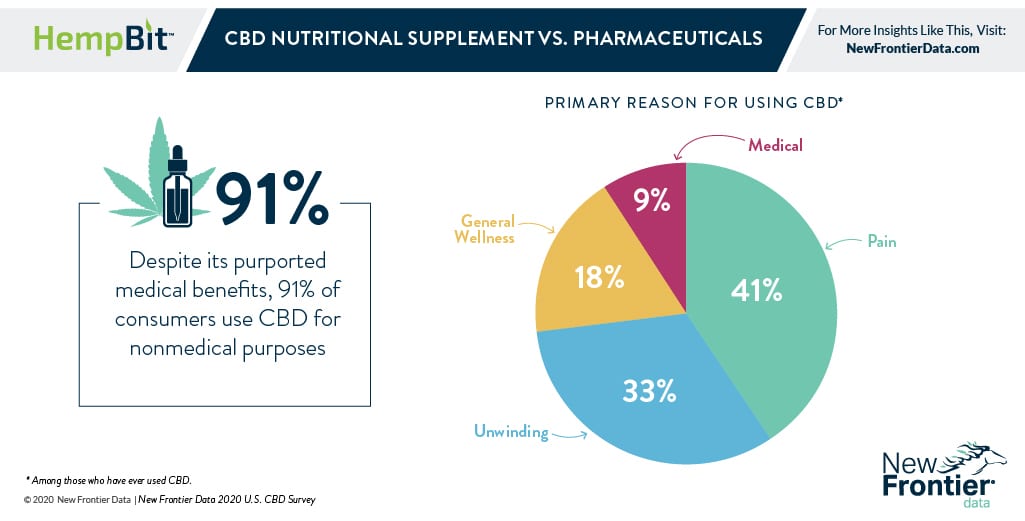
As the FDA continues to grapple with CBD, a key point of consideration will be how people are currently using it. While discussions about CBD concern the substance’s medical effects, the fact is most consumers do not use CBD for medical reasons.
According to the U.S. CBD Consumer Report: Archetypes and Preferences, Volume 1., approximately 91% of CBD users report using it primarily for nonmedical purposes, including pain management and general relaxation.
Not content to wait for the FDA’s decision, several federal lawmakers have acted by introducing a bill to regulate CBD.
Introduced by U.S. Reps Kurt Schrader (D-Ore.) and Morgan Griffith (R-Va.), the Hemp and Hemp-Derived CBD Consumer Protection and Market Stabilization Act of 2020 (H.R. 8179) would allow CBD and other nonintoxicating hemp derivatives to be sold as dietary supplements, while requiring manufacturers to comply with current regulations governing dietary supplements.
Though the measure has been referred to the House Energy and Commerce, given how close it is to the November elections, it is unlikely that lawmakers will act on the measure before a new Congress is sworn in next year.


