Confusion About CBD Legalities Stymies Manufacturers, Consumers Alike
By William Sumner, Hemp Business Journal Contributor
When President Trump signed the 2018 Farm Bill into law, many legal ambiguities which plagued the hemp industry were eliminated. Hemp is no longer considered a controlled substance, and states are free to establish industrial hemp programs for commercial purposes. Yet, issues surrounding cannabidiol (CBD) have only deepened as states continue to grapple with the question of how to regulate the increasingly popular substance, and complexities stemming from its sourcing from both marijuana and hemp.
Under the Farm Bill, the United States Food and Drug Administration (FDA) has the authority to regulate hemp-derived products, including CBD. However, the FDA has yet to develop any policy regarding the use of CBD as either a supplement or food additive.
That lack of policy guidance has effectively consigned CBD to a legal gray area in which it is neither a controlled nor regulated substance. Subsequently, many manufacturers have interpreted the gray area as a green light for them to infuse everyday food products and beverages with CBD. Across the country, restaurants have started offering things like CBD-infused pizza, and CBD-infused products have been popping up on the shelves of retail stores.
In the absence of clear guidance by the FDA, it has been left to individual states to tamp down on the growing enthusiasm for CBD-infused products. For example, in Maine, health officials with the state’s Department of Health and Human Services have issued an order for businesses to remove any edible food product that contains hemp-derived CBD.
States like New York have similarly issued orders to crack down on restaurants and bakeries offering CBD-infused foods. While critics claim that states are overreacting to the influx of CBD products, state officials have justified their actions by pointing out that the FDA has not deemed CBD to be a safe food additive.
For its part, the FDA remains cognizant of its regulatory responsibilities and the influx of CBD products. In a statement released when the Farm Bill was enacted, the FDA reiterated its authority to regulate CBD products, and pledged to hold public hearings to gather more information about CBD.
“We’re aware of the growing public interest in cannabis and cannabis-derived products, including cannabidiol (CBD),” the statement reads. “This increasing public interest in these products makes it even more important with the passage of this law for the FDA to clarify its regulatory authority over these products.”
No public hearing has yet been scheduled, and no regulatory guidance provided, frustrating each manufacturers, consumers, and law enforcement officials.
In response to the FDA’s lack of action, a group of bipartisan lawmakers led by Maine Congresswoman Chellie Pingree have penned a letter to FDA Commissioner Scott Gottlieb. In it, lawmakers ask Gottlieb three questions: When will the FDA provide guidance on CBD?, did the FDA advise states like New York to act against CBD-infused food products?, and when will the FDA hold a public hearing on CBD? The letter gives Gottlieb until February 22 to respond.
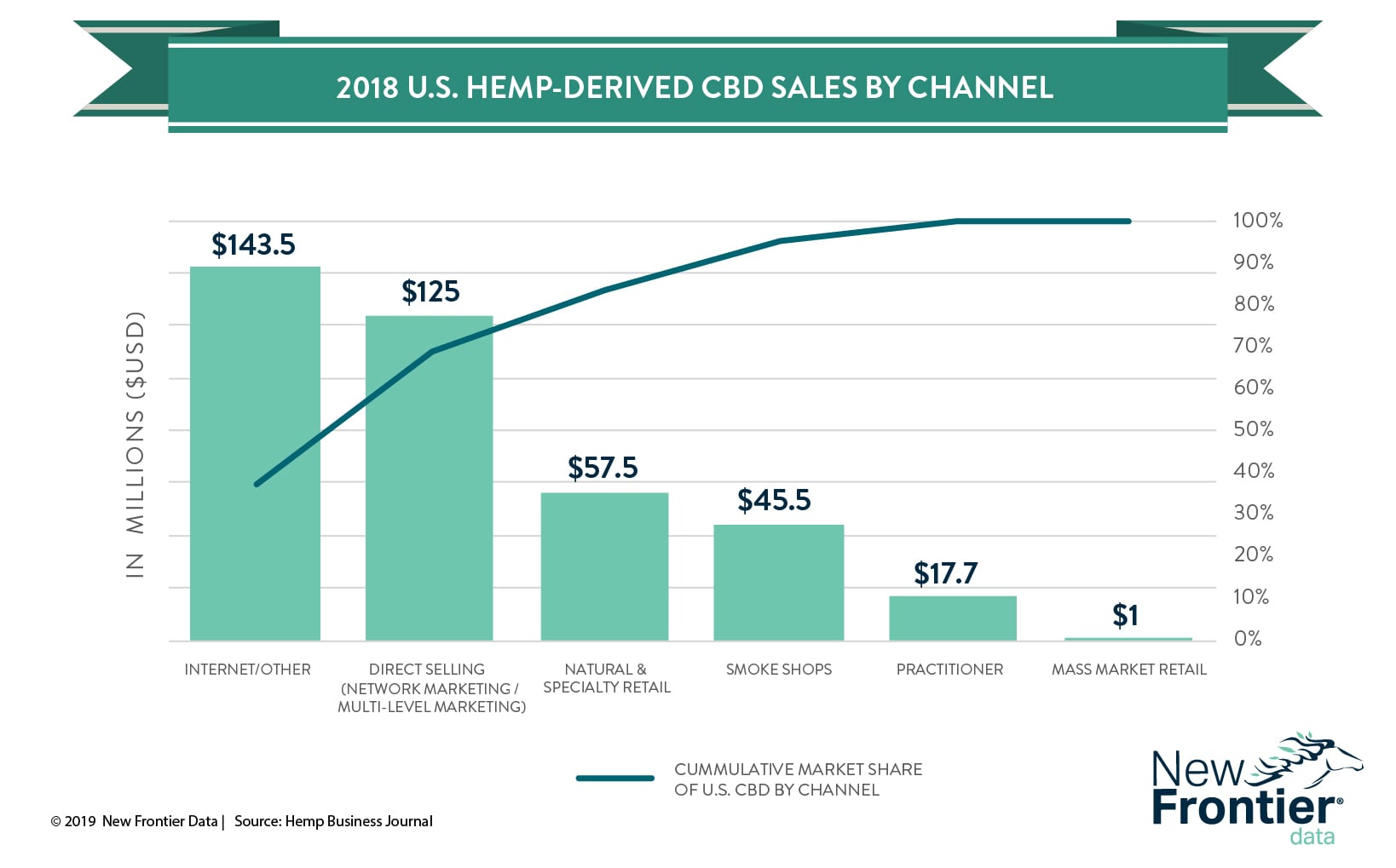
Despite the confusion surrounding the use of CBD as a food product, the hemp-derived CBD market as a whole is thriving. For the most part, the recent state-led crackdown on CBD products has primarily affected brick-and-mortar retail shops, which comprise only a small segment of the CBD market.
The Hemp Business Journal estimates that the vast majority of 2018 CBD-related sales came from the internet ($143.5 million) or direct selling channels ($125 million). In contrast, mass-market retail CBD sales accounted for roughly $1 million during 2018.
As the issue evolves, and clarity is made forthcoming from state and federal regulators, keep referring to the Hemp Business Journal and New Frontier Data for regular updates.
William Sumner
William Sumner is a writer for the hemp and cannabis industry. Hailing from Panama City, Florida, William covers various topics such as hemp legislation, investment, and business. William’s writing has appeared in publications such as Green Market Report, Civilized, and MJINews. You can follow William on Twitter: @W_Sumner.