Treading the Muddied Waters of CBD Standards for Marketing and Safety

By William Sumner, Hemp Business Journal
The U.S. Department of Agriculture (USDA)’s rollout of interim rules for the federal domestic hemp program provided some much needed guidance to the industry. While included were provisions governing hemp testing, license applications, and interstate commerce, noticeably absent from such was any guidance about cannabidiol (CBD), and how state and federal governments should regulate the chemical compound.
As outlined, the USDA shied away from issuing CBD regulations since the U.S. Food and Drug Administration (FDA) has primacy over the issue. Nevertheless, the need for solid CBD guidance from the federal is an ever-pressing issue, especially in light of the vaping health crisis which by November had affected 2,172 Americans in 49 states, resulting in 42 deaths.
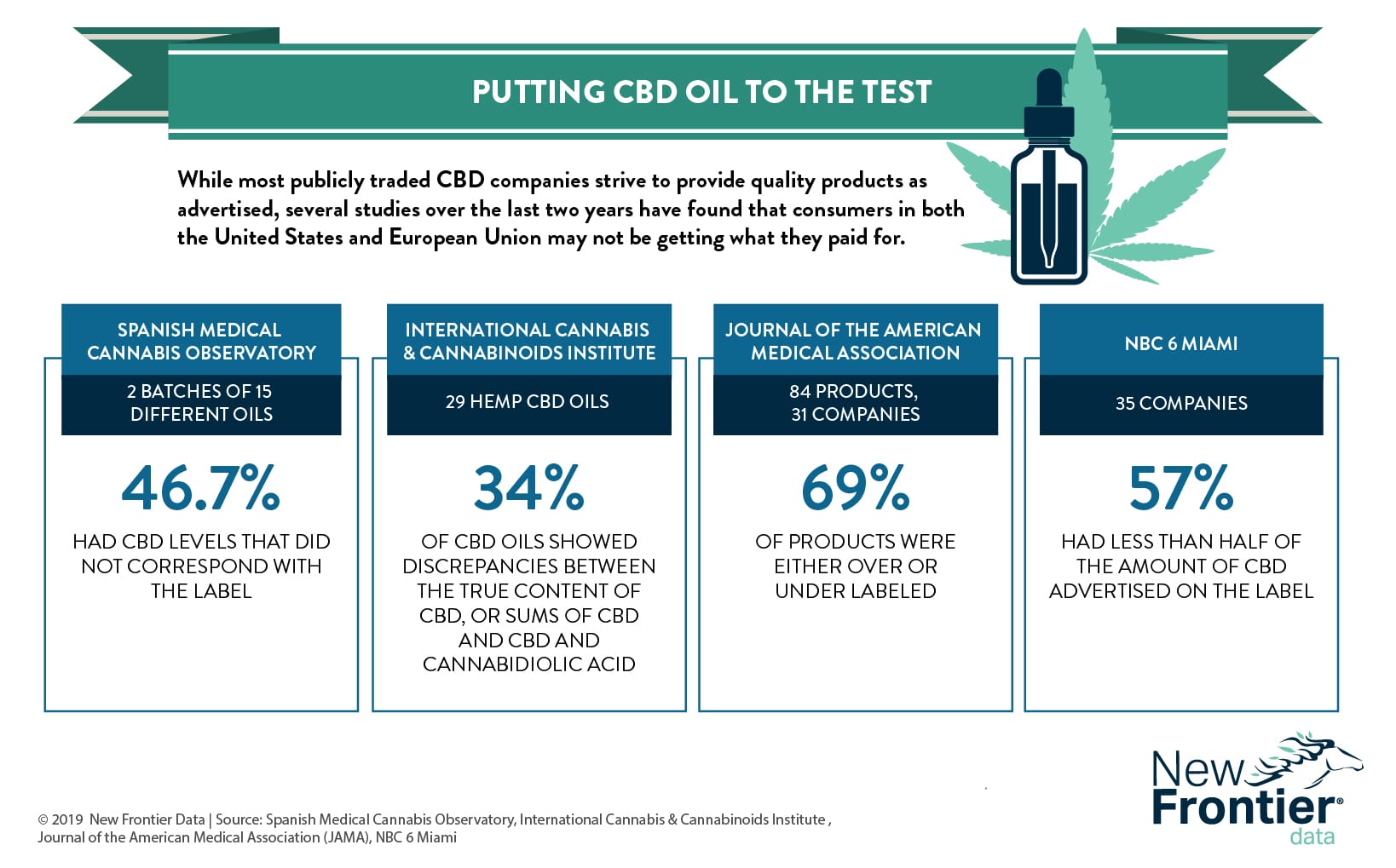
In addition to concerns directly related to vaping, there are others about product quality and consistency. While most publicly traded CBD companies strive to provide quality products as advertised, several recent studies have consistently found that consumers (whether in the United States or the European Union) have not been getting what they paid for.
In a 2017 study published in the Journal of the American Medical Association (JAMA), researchers conducted lab tests on 84 CBD products purchased from 31 companies. They found that 43% of the tested samples were under-labeled, meaning that the products contained more CBD than disclosed on the product packaging. Another 26% of the tested CBD products were over-labeled (i.e., containing less CBD than advertised), while less than a third (31%) were labeled accurately.
Those products least often correctly labeled were vaporizer products, while the products most accurately labeled were oils.
Other investigative reports unearthed similar results: A test of 35 CBD products from seven different companies revealed that 20 (57%) of them had less than half of the CBD as advertised, while five (14%) contained no CBD at all.
Multiple CBD products sold in the European Union (EU) also failed scrutiny.
Through the efforts of Americans for Safe Access and its Patient Focused Certification (PFC) program, the medical cannabis advocacy organization tested 29 CBD-based oils for quality and authenticity. They found that 20 (69%) out of 29 oils tested positive for a carcinogen called polyaromatic hydrocarbon. Approximately 34% of the oils tested were either under- or over-labeled.
Hoping to rein in the CBD market, the European Food Safety Authority (EFSA) issued regulations classifying CBD as an unauthorized novel food. A novel food is defined as one “that had not been consumed to a significant degree by humans in the EU before 15 May 1997, when the first regulation on novel food came into force.”
Under the classification, producers selling CBD products on the European market must attain pre-market authorization and prove the safety of those products. Though the Novel Food Catalogue is not legally binding, EU member states like Germany have started adopting the EFSA’s CBD definition.
Meanwhile, regulators in the United States are still struggling with how to legislate CBD. Over the summer, the FDA held a public hearing to collect public comment about CBD. Despite having pledged issuance of a regulatory update by early fall, no such report has materialized.
Conversely, in a September letter to FDA Acting Commissioner Ned Sharpless, lawmakers led by Minority Leader Sen. Chuck Schumer (D-NY) urged the agency to speed up its regulatory process.
In the interim, the FDA continues to send warning letters to CBD companies making unverified medical claims, sending seven warning letters to marketers so far this year.
As underscored by the vaping crisis, the burgeoning swell of unregulated CBD products flooding the market critically heightens the need for meaningful regulation of the CBD market. Until the FDA releases its draft rules, it remains incumbent upon both consumers and investors to conduct due diligence when considering a CBD-based product.
William Sumner
William Sumner is a writer for the hemp and cannabis industry. Hailing from Panama City, Florida, William covers various topics such as hemp legislation, investment, and business. William’s writing has appeared in publications such as Green Market Report, Civilized, and MJINews. You can follow William on Twitter: @W_Sumner.



