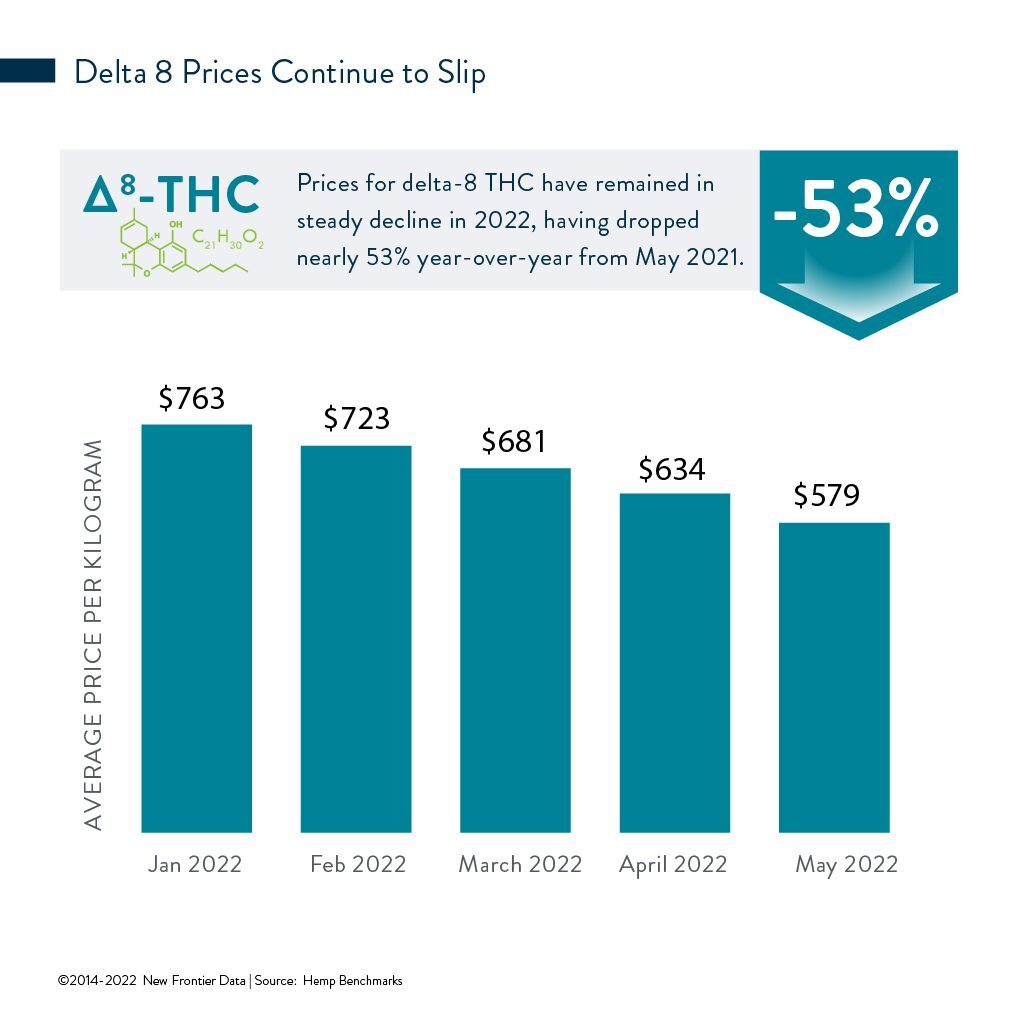
A Federal Decision and More Dysfunction Over Delta-8 THC

Smart and New Frontier Data Partner to Provide Advertisers Access to 160M+ US Cannabis Consumer Profiles
May 31, 2022
Are NSAIDS More of a Pain Than They’re Worth?
May 31, 2022By J. J. McCoy, Senior Managing Editor, New Frontier Data
Ah, the law of unintended consequences: If anyone can create a snafu quite like a dysfunctional Congress, it’s a federal appeals court, especially when cannabis is involved.
Latest case in point: A three-judge panel in San Francisco has found that delta-8 is legal under the federal 2018 Farm Bill, which legalized hemp cultivation and opened the market’s gates for cannabidiol (CBD) and other hemp extracts. Even in states which still prohibit cannabis whether for medicinal or adult-use consumption, the intoxicating, hemp-derived cousin of delta-9 THC has become widely available while operating in legal but unregulated gray areas.
Dawn of Delta-8
Commonly found as gummies, vape cartridges, tinctures, and other products, delta-8-THC can be found in gas stations, convenience stores, tobacco shops, and cannabis dispensaries throughout the U.S. and beyond – often with no age restrictions. Consumers commonly find delta-8 THC to present pleasant potency/efficacy. They do not experience quite the psychoactivity or impairment that they might from delta-9 THC, and it does not last as long. The result is a generally lighter experience for consumers.
Among producers, its popularity stems from when an oversupply of CBD extracted from U.S.-grown hemp caused CBD prices to plummet. Producers saddled with the glut looked for ways to spin CBD into something profitable. With some pretty simple chemistry and the internet, producers got creative about converting CBD to delta-8 THC.
Consumers looking to relieve stress and anxiety – and especially those either not wanting to use traditional cannabis products or those living where cannabis remains prohibited – were happy for the inexpensive, increasingly ubiquitous, and less-intense “cannabis light” being made available.
But lacking regulatory oversight or even limited laboratory testing, most products sold as delta-8 THC are not truly pure. Such products typically contain a high percentage of delta-8-THC and small amounts of other cannabinoids (including delta-9-THC) with other byproducts. Some of the cannabinoids are not naturally found in cannabis. In most cases, little to nothing is known about health effects from the impurities.
Law Provides for Hemp
On May 19, the U.S. Ninth Circuit ruled that the 2018 Agriculture Improvement Act (i.e., the 2018 Farm Act) removing most restrictions on hemp also legalized the extracted, hemp-derived ingredient delta-8 THC, which has psychoactive and intoxicating effects like the marijuana ingredient delta-9 THC, which remains federally prohibited. The ruling effectively lifts recent bans on hemp and any derived products that contain less than 0.3% of delta-9 THC by dry weight.
As it happened, the ruling also came two weeks after the FDA issued a consumer update warning the public about safety concerns regarding delta-8 THC products.
Regardless, the appeals court decided, “the act is silent with regard to delta-8 THC,” adding that “plain statutory text” compelled its conclusion that delta-8 THC products are lawful. The opinion authored by Judge D. Michael Fisher added that if Congress “inadvertently created a loophole legalizing vaping products containing delta-8 THC, then it is for Congress to fix its mistake.”
Fisher (temporarily assigned to the Ninth Circuit from the federal appeals court in Philadelphia), was appointed by President George W. Bush. He was joined by Judges Andrew Kleinfeld, appointed by President George H.W. Bush, and Mark Bennett, a Trump appointee. In practical effect, the court’s ruling federally legalizes a psychoactive cannabinoid about which relatively little is known, while keeping the well-studied delta-9 THC illegal though it has been thoroughly studied for decades and through thousands of subjects and research protocols.
Over the past few years, products made with the cannabinoid delta-8 THC have become widely available to consumers from coast to coast, gaining a reputation as “cannabis light”. Like its chemical relative delta-9 THC (commonly consumed for a “classic marijuana high”), delta-8 THC provides psychoactive effects, though not as potently.
FDA Concerns
On May 4, an FDA consumer update warned of “Five Things to Know about Delta-8 Tetrahydrocannabinol – Delta-8 THC”, including a lack of research or evaluation, adverse reports about its use, its psychoactive and intoxicating effects akin to delta-9 THC, risks from unchecked and potentially harmful contaminants through chemical synthesis, and unlawful or irresponsible packaging which may attract children.
“A combination of factors has led the FDA to provide consumers with this information,” the agency noted. “These factors include: an uptick in adverse event reports to the FDA and the nation’s poison control centers; marketing – including online marketing of products – that is appealing to children; and concerns regarding contamination due to methods of manufacturing that may in some cases be used to produce marketed delta-8 THC products.”
Delta-8 THC’s Trending
Last summer, Fortune magazine interviewed John Kagia, New Frontier Data’s Chief Knowledge Officer, about delta-8 THC as a “phenomenon that has taken the industry quite by storm.”
New Frontier Data first examined the issues about its legality and marketability in a March 2021 blog detailing its legal loophole and prospects for disruption.
Producers Take Heart
For now, Nicole Brown is choosing an optimistic view. From her company’s perspective, the chief innovation officer (CIO) for Open Book Extracts in North Carolina says, “on the positive side, we’ve all seen the proliferation of products on the market over the last 12-18 months, and saw things potentially going in a downward direction with up to 19 states banning it.”
With the court ruling now turning that the other way, “maybe now it does become the cannabinoid of interest to solve some of the issues in the market, for both therapeutic as well as recreational benefits. If done the right way, it can be a very positive outcome for the market, with access to well-studied, quality materials to let us see what its role might be in the markets.”
That remains a big “if”, though: “Conversely,” Brown says, “the levels of confidence of purity and quality have not been what we’d like to see. Rather than just make it legal, let’s put regulations and parameters in place. Maybe that’s coming. That would make us feel a lot better about the roles that this could play.”
Legal Questions Remain
Attorney Garrett Graff with Denver-based Moye White, specializing in cannabis and hemp, is perhaps even more sanguine. “I’m not sure that this opinion will change the cannabinoid landscape all that much,” he says. “Companies selling hemp-derived cannabinoids such as delta-8 THC were already emboldened to do so, despite the regulatory uncertainties – these companies didn’t necessarily need the court’s opinion to feel emboldened to do so. That said, even with this court’s position under federal law, this opinion does not necessarily change the laws of states which have affirmatively regulated (or prohibited) delta-8 THC and other intoxicating cannabinoids. Those state-level regulations still present potential legality challenges.”
More uncertainty, Graff notes, exists regarding trademarks and copyrights.
“The opinion addresses the ‘lawfulness’ of delta-8 THC under federal law in the context of trademarks and copyrights,” he said. “As to CBD, the United States Patent and Trademark Office (USPTO) historically has cited lawfulness both under DEA regulation (i.e., the old argument that hemp/CBD is a controlled substance), but it’s also FDA’s position that CBD is not a permissible ingredient, and thus violates federal law.”
Yet, Graff adds, “the court did not explore the FDA position – if it had, I imagine the court would have found the subject trademarks to be unenforceable as a violation of federal law, by virtue of delta-8 THC not being a permissible ingredient in the eyes of FDA.”
While Graff considers the court decision as favorable for the hemp industry, the short-term impacts on the markets will be slight. “I think that this court order will ultimately be used rhetorically for the hemp industry,” he said, “though I think that the scope is far more narrow than is recognized.”
At the end of the day, he continued, “synthesis itself should not be demonized (vitamin C is conducted by synthesis), but they want to make sure that the materials and ingredients being used are safe. There should be evaluation whether there are thresholds, and whether they should be sold by a regulated dispensary or a gas station. Those are questions for the FDA, not the DEA. The court only addresses the FDA side of things.”
The opinion seems ultimately bound to spur further action both at the federal and state levels regarding appropriate regulations for all cannabinoids, including intoxicating ones such as delta-8 THC.
Until Congress and other government officials can grasp and ably address issues like maintaining the chain of custody for the plant, or remedying the illicit market’s undercutting the legal cannabis industry across the board (since the costs of obtaining licensing, using quality materials, and conforming to standards, etc., are borne entirely by legitimate companies), consumers will not receive the quality and safety they expect, the industry will struggle to gain and maintain public confidence, and the markets will not mature as effectively or profitably as either its consumers or stakeholders expect or aspire.
“What does this do for existing companies working with minor potential intoxicating cannabinoids? They already present the risk appetite,” Graff noted. “They may well keep doing what they’re doing, with the existing tension between marijuana companies and hemp companies. The clamoring will get louder and louder about how to marry those two sides of the industry.”