Natural and Specialty Retail Hemp-Derived CBD Sales Projected to Grow by More Than 600% by 2022

Cannabis Potential $5 Billion Economic Impact onto North American Tribal Communities
August 23, 2018
Growth Strategy for the Green Ocean
August 26, 2018By: William Sumner
One of the fastest growing markets in the United States is the cannabidiol (CBD) industry. From joint pain to skincare, Americans are using CBD to help treat everyday maladies. Natural products and specialty retailers are reaping the rewards of this increased interest.
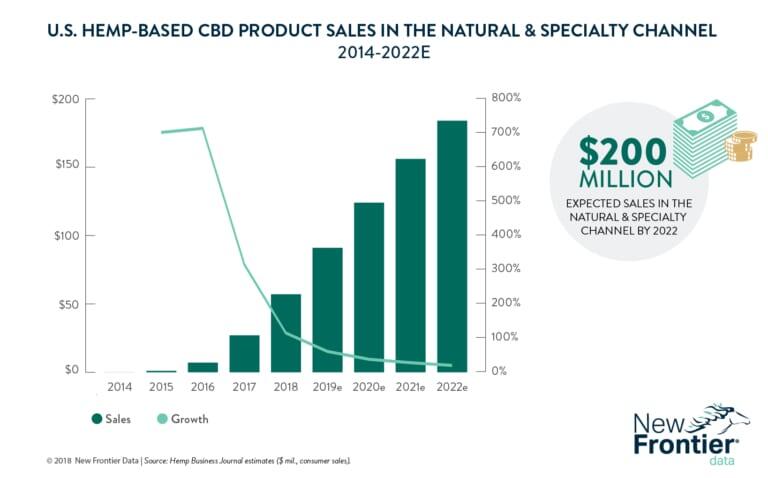
By 2022, the Hemp Business Journal estimates that hemp-based CBD sales will reach $646 million, with more than a quarter of those sales coming from Natural and Specialty retailers.
In 2017, hemp-based CBD sales increased by nearly 50%, growing from $129 million in 2016 to $190 million. Roughly 60% of those sales came from the internet, with another 21% coming from smoke shops, 14% from natural and specialty retail, and only 5% from healthcare practitioners. Although the vast majority of sales came from the internet or other direct sources, those numbers are expected to drop to less than 30% by 2020 as more traditional retail and pharmaceutical options become available.
Natural and Specialty retail sales, on the other hand, are expected to increase by more than 600% by 2022; rising from approximately $26 million in 2017 to $184 million.
Generally, hemp-based CBD is labeled in one of two ways, both of which are meant to showcase specific aspects of the product. The first labeling method is intended to emphasize that the product contains CBD. One example of this labeling method comes from CV Sciences, which sells a line of products called PlusCBD Oil.
The second labeling method is meant to showcase that the product is derived from hemp. For example, the company Charlotte’s Web calls its line of products a “premium hemp extract supplement.” Companies will often highlight that their CBD products are derived from hemp for legal purposes, as CBD can also be extracted from cannabis, which is still federally illegal as a Schedule 1 drug.
Companies are also incentivized to label their products as hemp-derived supplements to help avoid the ire of the U.S. Food and Drug Administration. Although the supplement market is largely unregulated, the FDA will often crack down on supplements making medical claims, especially in the CBD industry where products have been mislabeled.
Since 2015, the FDA has sent dozens of warning letters to companies that manufacture CBD products for making unsubstantiated medical claims, selling products that do not contain the amount of CBD that the company claims it does, and for violating current good manufacturing practice (CGMP) regulations.
Most recently, the FDA sent out a warning letter to Signature Formulations for violating CGMP regulations and making unsubstantiated medical claims about their products.
Given the predicted trends in the natural and specialty retail market, the next phase of growth for the hemp-based CBD market lies in brands distributing to large-scale national specialty retailers and penetrating into mass market channels.
To date, most national specialty retailers are staying on the sidelines, adopting a wait and see approach until the FDA makes its stance clear on CBD, which will likely be later this year after the DEA schedules Epidiolex and the new CBD drug officially goes to market.
The only national retailers to enter the hemp-derived CBD industry are Lucky’s Market and Fresh Thyme. With more than 3,000 stores nationwide, GNC is the largest natural and specialty retailer in the U.S. followed by Vitamin Shoppe, Whole Foods Market and Natural Grocers. With such explosive growth in hemp-derived CBD sales through natural and specialty retail channels, readers should continue to keep an eye on the 2018 Farm Bill, which, if passed, will likely give large retailers more confidence to carry hemp-derived CBD products.
By: William Sumner