Additional Revenues from Potential State Legalization
June 24, 2018Despite Recognition of Injustice, Arrest Rates for Cannabis Remain Unequal
July 8, 2018
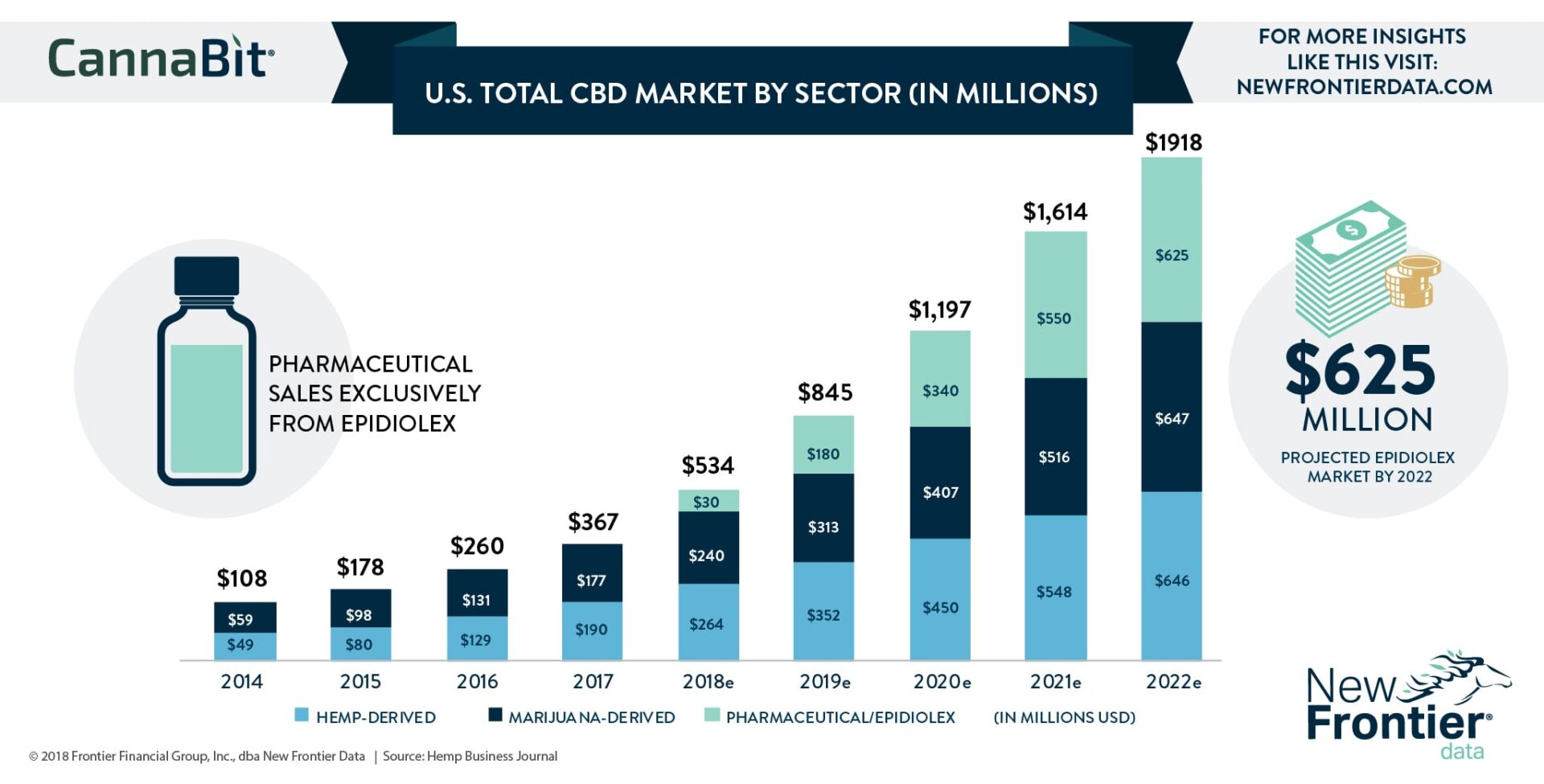
- The FDA last week approved UK-based GW Pharmaceuticals’ Epidiolex, a “pure plant-derived CBD” extract shown to drastically reduce seizures in children with epileptic syndromes.
- Marking the FDA’s first-ever approval of a cannabis-derived pharmaceutical product, the DEA has 90 days (until September 23) to schedule Epidiolex, a landmark decision being carefully scrutinized by the industry at large.
- Sales of Epidiolex will effectively open the pharmaceutical CBD market; the Hemp Business Journal (a division of New Frontier Data) forecasts the drug to generate between $15 million and $30 million in sales through 2018, and $180 million in 2019.
- HBJ expects the DEA to schedule Epidiolex as a Schedule IV drug and recommend that CBD be rescheduled from a Schedule I drug.
- Continued regulatory action by the FDA is expected against illicit claims and sales of unapproved, CBD-related products marketed with unproven medical claims.