Consumer Safety Regulations Vary Per Cannabis-Infused Options
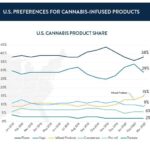
U.S. Preferences For Cannabis-Infused Products
April 26, 2020
California Cannabis Cultivation Licensing Update
May 3, 2020By Rob Kuvinka, Data Science Manager, New Frontier Data
Last week’s CannaBit examined how manufacturers are responding to maturing consumer demand through technical innovation. Investments in manufacturing advancements including water-based emulsion are increasing bioavailability while reducing onset times and improving the taste of cannabis-infused products.
Beyond satisfying consumers, however, cannabis-infused products are also evolving to address new consumer-safety regulations.
In early 2019, the European Commission announced that ingestible products containing cannabis would be categorized as “novel foods”, presenting regulatory barriers against sales of CBD edibles and beverages across the continent. However, few member states (e.g., Austria and Spain) effectively enforced the mandated safety evaluations. Conversely, in February 2020, the U.K. became the world’s first nation to define a clear regulatory path to selling fully compliant CBD ingestibles. Effective by March 2021, its Food Standards Agency (FSA) will require testing and toxicology reports on all the compounds found within novel food CBD products.
The consequences of the U.K. regulations will likely impact CBD products worldwide. With dozens of unknown or poorly understood compounds in full- and broad-spectrum oils, testing requirements will favor development of isolate products. The relatively burdensome application process is also likely to leave many current CBD manufacturers sidelined, while those receiving approval will find themselves in good stead, free to operate in a less competitive and fully legal environment.
Meanwhile, technicalities in the manufacturing process seem bound to leave cannabis-infused cosmetic and topical products occupying more of a legal gray zone for EU markets.
Across the pond, the United States Food and Drug Administration (FDA) has been comparatively reticent on the federal level regarding the broader CBD market. Consequently, several states have issued their own guidance and rules to provide clarity to business stakeholders. As of this month, 15 states have made CBD legal by statute, another 15 mandate labeling requirements, 18 more have no laws on the books, and three ban CBD altogether. Labeling, testing, and registration requirements vary state-by-state regarding which types of cannabis-infused products are permitted. Oregon, for instance, recently ruled that no beer or other alcoholic beverage may contain CBD or THC.
Emulsion technology, too, is subject to variable regulatory constraints. The FDA has been consistent on ensuring that the safety of ingredients in emulsions is understood, but some emulsion companies press the issue further by making what they claim to be nanoemulsions. Any products at the nanoscopic scale will need to prove their safety as nano-sized components, in addition to proving the safety of the ingredients in combination. Such scrutiny may raise a potential red flag for manufacturers and processors.
Further analysis about the market performance of cannabis-infused products and the impacts of consumer safety regulations is available in New Frontier Data’s Cannabis-Infused Products Report Series: U.S. Consumer Experience and Demand, in partnership with SōRSE Technology.