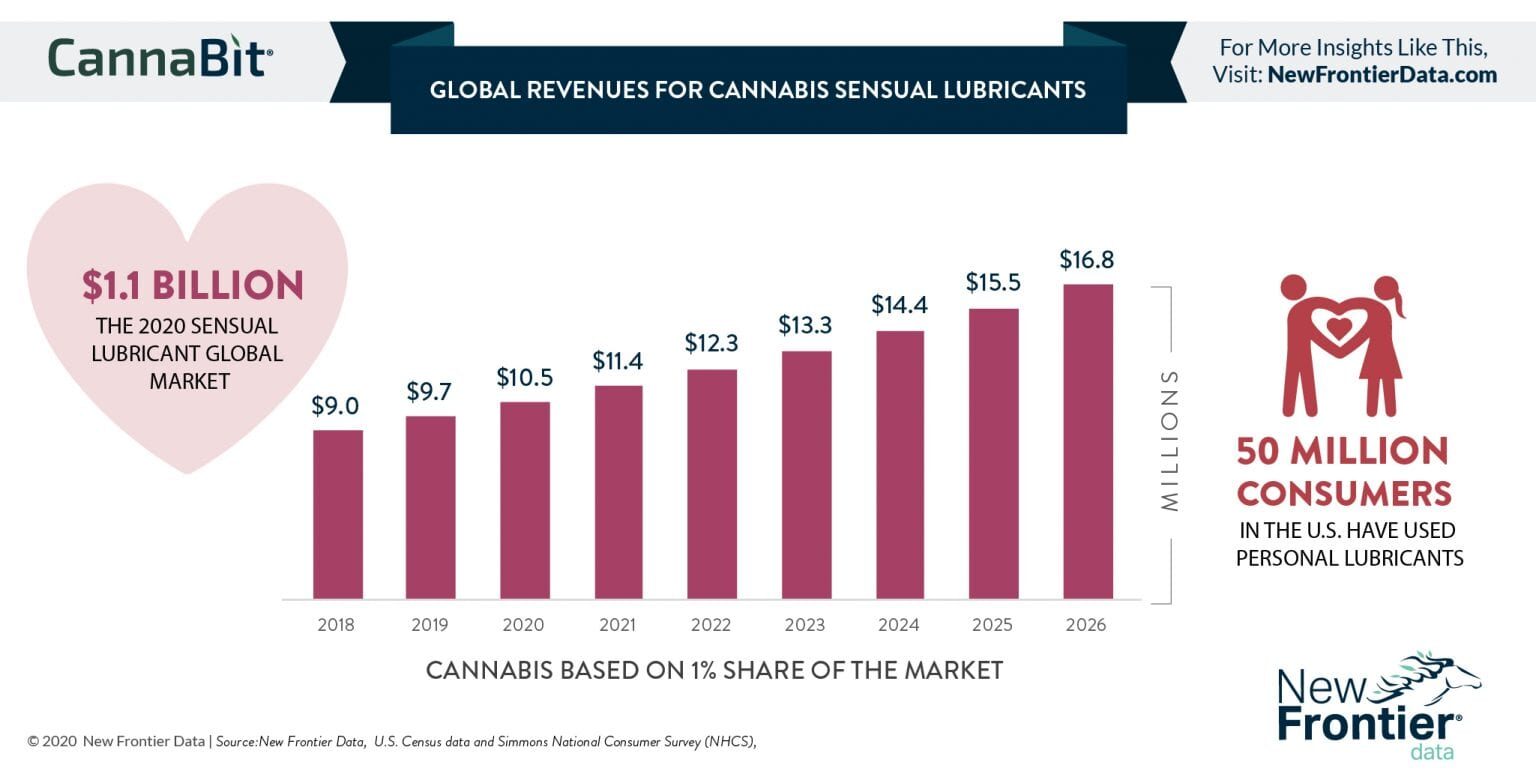
Global Revenues For Cannabis Sensual Lubricants

For Valentine’s Weekend, Sensual Lubricants Add to Cannabis Options
February 16, 2020
U.K. Favorite CBD Product Type (Among CBD Consumers)
February 23, 2020- The global market for sensual lubricants projects to $1.1 billion for 2020, an estimated $350 million of it in the U.S.
- Nearly 50 million U.S. consumers have reportedly used personal lubricants.
- Beyond use as a sexual aid, sensual lubricants are used to address conditions including vaginal dryness, menstrual cramps, or painful intercourse, along with conditions among pre- and post-menopausal women, including uterine prolapse.
- In the U.S., personal lubricants are regulated by the FDA, which categorizes them with medical devices; manufacturers must comply with good manufacturing practices and receive 510(k) medical device clearance
- The FDA approval process generally extends between 6 months to 3 years.
Receive the best hemp news in the industry delivered to your inbox every week!